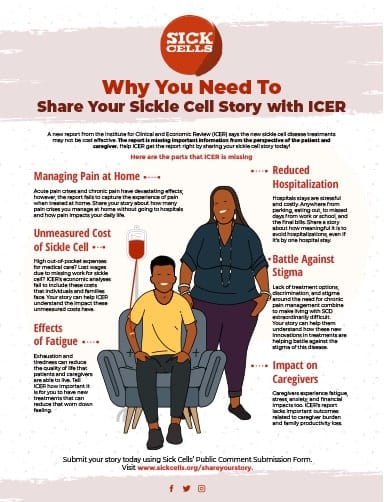
Understanding the “value” of sickle cell treatments has become a priority across the sickle cell disease (SCD) community. Value assessments, or health economic assessments, can advise whether a new treatment should be used, and if so, which patients are most likely to benefit from it.
Recommendations stemming from value assessments are used to inform the decisions of clinicians, patients, and payers and can impact the coverage and access for new treatments. At Sick Cells, we focus on improving value assessments for sickle cell disease through:
- A transparent and collaborative approach
- representation of patient and caregiver perspectives
- high-quality databases that adequately account for the diversity of SCD patients
- methods that support equity
Sick Cells works with patients, researchers, health economists, payers, and providers to find the right approach to measuring cost and value for SCD.